Conference Information
About Conference
The world healthcare has been evolving in a rapid phase and there have been numerous researches and trials conducted to discover, develop and test the new drugs to improve healthcare facilities around the globe. During this process researchers do encounter various hurdles. It becomes very much important to discuss these challenges with the research community and address the same. Clinical Research 2020 is one such platform where group the young researchers and few eminent researchers to discuss on the recent happenings in the subject. This event also serves as a mentorship platform for the young researchers who are looking to boost their research career.
Pulsus Conferences welcomes the Elite Scientists, budding researchers, practitioners, Physicians and other medical practitioners to come and join our scientific community in “International Conference on Clinical Research and Case Reports” which is scheduled to be held in Paris, France during June 29-30, 2020.
Scientific Session
Session 01: Clinical Data Management
Clinical data management is a process in clinical research that encompasses generation of reliable and statistically high-quality data obtained from clinical trial studies involving human participants. Clinical data management involves various procedures such as database design, data entry, data validation, data extraction, database locking, case report form designing, case report form annotation and many others. Clinical Data Management is the procedure of accumulation, cleaning, coordination and administration of subject information in consistence with administrative guidelines. CDM additionally underpins the direct administration and examination of studies over the range of clinical research.
Clinical Data management Conferences | Clinical Research Conferences | Case Reports Conference
Session 02: Pharmacoepidemiology
Pharmacoepidemiology is the examination of the use and effects of drugs in significant amounts of people. It is developing order that applies epidemiological strategies to consider the use in people. So also, as the term construes, pharmacoepidemiology solidifies clinical pharmacology with the investigation of disease transmission. Pharmacoepidemiology updates the valuable review on topics related to Pharmacoepidemiology, Pharmacokinetics, Adverse prescription reactions, Public prosperity, Epidemiologic methodologies, Medication adherence and Drug security.
Clinical Trails Conferences | Pharmacoepidemiology conferences | Clinical Research Conferences
Session 03: Pharmacovigilance and Drug Safety
Pharmacovigilance is characterized as the science and exercises identifying with the recognition, appraisal, comprehension and counteractive action of unfriendly impacts or some other medication-related issue. Medication wellbeing identifies with the potential for unfavourable impacts identified with the organization of medications. Endeavours to set up the secure profile of medications start right off the bat in their improvement, with in vitro, and in vivo harmfulness testing, and proceed through clinical preliminaries paving the way to sedate endorsement and following endorsement in explicit post-promoting studies or general pharmacovigilance endeavours.
Pharmacovigilance Conferences | Clinical Trials Conferences | Clinical Research Conferences
Session 04: Globalization of Clinical Trials
The globalization of clinical research is a moderately on-going marvel, in which a considerable lot of these examinations are occurring on a worldwide scale, with a noteworthy increment of clinical preliminaries in creating nations. Worldwide pharmaceutical organizations are doing the expanding number of preliminaries at locales in the creating scene, notwithstanding destinations in rich nations. In such manner, we are obliged to keep guaranteeing that the structure, oversight, and execution of the investigations are completely tended to and meet both legitimate and moral gauges. The procedure of globalization of clinical preliminaries, in this way, can be beneficial in light of the fact that, for instance, it provides for access to new medications to members; in any case, it requires discourse and the checking of moral inquiries related for the most part to guaranteeing the uprightness, welfare, and security of the examination member; to the edges of reference of bioethics, for example, self-rule, nonmaleficence, value, equity and decency.
Clinical Trials Conferences | Clinical Research Conferences | Case Reports Conferences
Session 05: Ethics in Clinical Trials
Morals in clinical research centres to a great extent around distinguishing and actualizing the worthy conditions for introduction of a few people to dangers and weights to support society on the loose. Moral rules for clinical research were planned simply after disclosure of obtuse conduct with members amid research tests. The essential duty of the Ethics Committee is to guarantee a free, skilful and convenient survey of every single moral part of the undertaking proposition got so as to defend the poise, rights, wellbeing, and prosperity of all genuine or potential research members. An all-around recorded educated assent process is the sign of any moral research work. Educated assent regards person's independence, to partake or not to take part in research.
Clinical Trials conferences | Medical Case Reports | Clinical Research Conferences | Clinical Case Reports
Session 06: Clinical Research on Different Diseases
Clinical Trials for various ailments and clutters are directed for assessing at least one mediations (for instance, drugs, medicinal gadgets, ways to deal with surgery or radiation treatment) for treating an infection, disorder, or condition and furthermore discovering approaches to keep the underlying improvement or repeat of a condition.. A few cases of the infections/ issue for which clinical preliminaries coordinating are Cardiovascular, Digestive structure, Respiratory system diseases and other parasitic, viral, bacterial and infectious afflictions.
Clinical Research Conferences | Clinical Trials Conferences | Clinical Case Reports | Medical Case Reports
Session 07: Risk Assessment and Management in Clinical Trials
Risk assessment is a precise procedure for distinguishing and assessing occasions that could influence the accomplishment of clinical investigation goals. A hearty hazard evaluation process in clinical preliminaries frames the establishment for a powerful hazard the board approach. It is tied in with adopting an all-encompassing strategy to distinguish every potential hazard and assess those dangers to evaluate the likelihood of event and effects. The basic step to risk assessment can include identify and assess risks, mitigate risks and review risks .Correct usage of a vigorous hazard appraisal process engages an investigation supervisory crew to all the more likely distinguish and assess the correct dangers for a clinical preliminary, all while keeping up the suitable controls to guarantee powerful and proficient quality lead, persistent wellbeing and administrative consistency.
Pharmacovigilance Conferences | Clinical Trials conferences | Clinical Research Conferences
Session 08: Outsourcing and Collaborative Research in Clinical Trials
Redistributing of clinical preliminaries demonstrates the US Food and Drug Administration (FDA) and associations with new issues around quality and obligations. By fittingly trading obligations to Contract Research Organizations (CRO) supporters can execute some potential issues. As help needs to demonstrate what specific obligations, they are trading to the CRO in creating. Anything that isn't especially depicted in creating is regarded to be held by the help.
Globalization, re-appropriating and growing flightiness of clinical preliminaries have made the goal of achieving overall quality testing. The quality, as planned by managerial examinations of the pro goals, bolsters/contract investigate affiliations and Institutional Review Board, has been of stress to the US Food and Drug Administration, as there has been not by any means any change in repeat and nature of fundamental insufficiencies.
CRO is an association shrunk by another organization to oversee and lead the organization's preliminaries, obligations, and capacities. A pharmaceutical organization procures an organization to do clinical trials, obligations, capacities, and that organization is called as CRO. CRO is altogether talented in dealing with complex medication advancement programs requesting new administrative and clinical methodologies and will have know-how in executing and acknowledging the fruitful finish of novel medication improvement tries. A CRO may give such administrations as biopharmaceutical advancement, biologic test improvement, commercialization, preclinical research, clinical research, clinical trials administration, and pharmacovigilance. CROs additionally bolster establishments, explore foundations, and colleges, notwithstanding government associations.
Clinical Research Conferences | Clinical Research Conferences | Clinical Case Reports | Medical Case Reports
Session 09: Patient Centric Clinical Trials
Organizations today are increasingly open and don't see patients as insignificant "subjects" who produce information, – however as educated partners whose cooperation may be "centre" to the general accomplishment of preliminaries prompting the development of the idea of "patient-centric trials." The spend has developed, yet the patient's needs are not being met. In this way, quiet driven medication advancement is presently turning into the model that the business is following. Today, patients know, innovation-driven, and educated driving the adjustment in attitude and way clinical preliminaries are being drawn nearer and led.
the direct, administration and examination of studies over the range of clinical research.
Clinical Research Conferences | Clinical Trials Conferences | Medical Case Reports | Clinical Case Reports
Session 10: Bioinformatics in Clinical Research
Clinical Bioinformatics can be characterized as "the clinical use of bioinformatics-related sciences and advancements to comprehend atomic instruments and potential treatments for human maladies". Being explicitly centred around clinical setting, CBI is portrayed by the test of coordinating atomic and clinical information to quicken the interpretation of learning revelation into compelling treatment and customized medication.
Clinical Trials Conferences | Clinical Research Conferences | Clinical Case Reports
Session 11: Post Marketing Surveillance
Post marketing drug reconnaissance alludes to the checking of medications once they achieve the market after clinical preliminaries. It assesses drugs taken by people under a wide scope of conditions over an all-encompassing timeframe. Such observation is considerably more prone to recognize beforehand unrecognized positive or negative impacts that might be related to a medication. Most of post marketing observation concern Adverse Drug Reactions (ADRs) checking and assessment.
PMS is led by different sorts of associations and offices, including pharmaceutical makers, colleges, government offices, privately owned businesses, and customer backing gatherings. The motivation behind directing PMS may vary, contingent upon the point of view of the people leading the observation.
Clinical Research Conferences | Clinical Trials Conferences | Medical Case Reports
Session 12: Drug Discovery and Development
Drug discovery and improvement together are the finished procedure of distinguishing another medication and putting up it for sale to the public. Disclosure may include a screening of chemical libraries, distinguishing proof of the dynamic fixing from a characteristic cure or configuration coming about because of comprehension of the objective. Improvement incorporates thinks about on microorganisms and creatures, clinical trials and eventually administrative endorsement.
Drug Discovery Conferences | Clinical Research Conferences | Clinical Trials Conferences
Session 13: Innovations in Clinical Research
Clinical research is witnessing some major innovations in clinical trial procedures that may include concept of patient-centric clinical trials, automation of clinical trial supplies, use of innovative encryption methods like Blockchain to ensure data security, exploration of more digital technologies among others. Moreover, as new medication investigate and advancement has achieved the container neck, the pharmaceutical business starts to concentrate on the scan for new or elective medications, for example, conventional Chinese prescription that can treat basic as well as dangerous infections. To guarantee that there is a high likelihood of accomplishment, progressed innovation as well as approach and creative reasoning of preliminary plans are essentially connected. The cutting-edge innovation/technique incorporate two-way translational procedure from seat to-bedside in translational research, miniaturized scale dosing approach for wellbeing assessment, and enormous information investigation for recognizing covered up clinical advantages of some test medications. Patient participation in drug development process also a vital and innovative approach in drug discovery and, ultimately, clinical trial studies.
Clinical Research Conferences | Clinical Trials Conferences | Clinical Trials Congress
Session 14: Clinical and Medical Case Reports
A case report is a strategy for presenting something new that has been picked up from clinical practice. It could be around a peculiar or viably cloud condition, an outstanding introduction or disorder of a known sickness, or a much another way to deal with a common condition. A case report gives the arranged report of signs, finding, treatment, and follow-up of an individual patient. Case reports may contain an estimation profile of the patient and acknowledge a certified part in the field of therapeutic research. Also, case reports can fill in as an early advised flag for the opposing effects of new cures, or the introductions of new and making ailments.
Clinical Case Reports | Medical Case Reports | Clinical Research Conferences | Clinical Trials Conferences
Session 15: Clinical Trials Conducts
A clinical trial is a research study involving human participants to provide information on drug or drug products, medical devices, new therapies, vaccines or diagnostic procedures. Clinical trial studies are conducted to determine whether new drugs, diagnostics or treatments are both safe and effective. After testing investigational new therapies or drugs in the laboratory and in animal studies, those with the most promising possibilities are moved into human clinical trials. There are four characterizations of clinical preliminaries. The fourth occurs after the FDA has cleared a medicine or treatment and continues following the security of the treatment. A clinical audit incorporates ask about using human volunteers (in like manner called individuals) that is proposed to add to restorative learning. Test potential prescriptions in human volunteers to see whether they should be embraced for increasingly broad use in the comprehensive network. A treatment could be a drug, therapeutic contraption, or biologic, for instance, a counteracting agent, blood thing, or quality treatment. By sharing in clinical preliminaries, individuals can't simply expect a progressively powerful part in their own specific social protection, anyway they can similarly get to new meds and help other individuals by adding to restorative research.
Clinical Trials Conferences | Clinical Research Conferences | Medical Research Conferences
Session 16: Site Management and Study Coordination
Market Analysis
Scope and Importance of Clinical Trials and Clinical Research
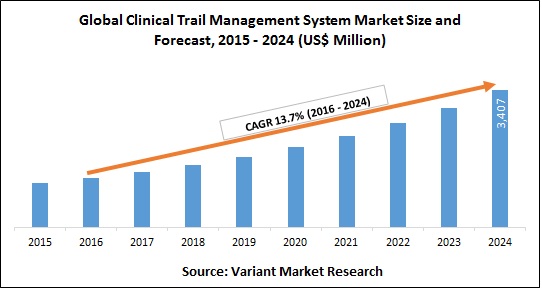
Globalization investment in new product development has increased in countries, positively impacting the market. The global market size of clinical trials and clinical research is expected to reach USD 68.9 billion by 2026, It is projected to expand at a CAGR of 5.7% during the forecast period. Key drivers impacting the market growth are the globalization of clinical trials, development of new treatments such as augmenting evolution in technology, personalized medicine and rising demand for CROs to conduct clinical trials.
Worldwide population has varied disease profile with emerging countries having the most diverse disease profile Growing prevalence of disease and new disease cases is expected to give a further boost to the clinical trial and research market.
The global clinical trials market size was calculable at forty four.2 billion is anticipated to expand at a CAGR of five.7% over the forecast amount. Factors like the economic process of clinical trials, innovations in treatments like personalized drugs, technological evolution, and demand for CROs to conduct clinical trials and research projected to drive the expansion.
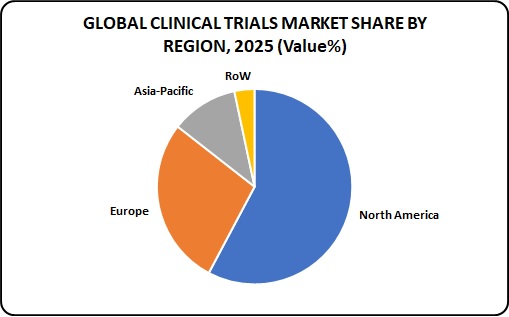
Heterogenous experience of CROs as compared to pharmaceutical company firms once it involves performing arts clinical trials in a big selection of geographies and development of medication in specific therapeutic areas, few factors account for the growing demand for the CROs in pharmaceutical section.
Why Marid: -
Madrid the capital of Spain is a prominent city originated in the middle of the Iberian Peninsula. Madrid is known for its Soccer publicity and achievements in Olympics and test matches. Other than more, Madrid has attractive tourist destinations such as Plaza Mayor, Templo de Debod, Madrid Cathedral, Plaza de la Villa, Puerta de Alcalá, Gran Vía, and etc,.Madrib is a hubspot of fashion and culture government,, education, entertainment, Infrastructure. Surrounded by Spanish speaking lovable people makes Madrid our next home, thus to conduct Clinical Trials and Research 2019 in this wonderful place on Earth in live and moist.
One beautiful thing is Manzanares River, Manzanares is said to be the heart of Madrid connecting railways gives a glimpse of crossing over the River in air.
Madrid exquisite Georgian engineering makes it a standout amongst Europe's most appealing capitals. Madrid is a moderately little and open city, little enough and safe, the Dart, give magnificent transport connects all through the city.
Clinical trials Societies and Associations in World: -
- European Society of Clinical pharmacy
- European Clinical Research Infrastructure Network
- European CRO Foundation
- Society for Clinical Data Management
- The European Association for Clinical Pharmacology and Therapeutics (EACPT)
- Association of Clinical Research Organization
- American Federation of Medical Research
- Indian Association for Statistics in Clinical Trials
- Pan Asian Clinical Research Association
- Institute of Clinical Research
Why you should attend Clinical Trials and Clinical Research 2019
- To sharpen your skills
- 10+ Keynote Speaker Session
- 50+ speaker faculty over 2 full days sharing Evidence
- 5+ Workshops
- 13 Interactive sessions
- The mixture of Health care units and Academia Delegates
- Meet Experts & Influencers Face to Face
- Networking Opportunities
- Learning in a New Space
- Break Out of Your Comfort Zone
- New Tips & Tactics
- The Energy of Like-Minded Individuals
- The Serendipity of the Random Workshop
- Invest in Yourself
Target Audience:
-
Professors and Students
-
Directors
-
Academic Scientists
-
Business entrepreneurs
-
Industry Professionals
-
Head of the department
-
Specialist in infectious diseases and tropical medicines
-
Medical specialist in Clinical trials
-
Specialist in clinical Clinical trials
-
Students and Professionals
-
Research institutes
-
Biotechnologist
-
Research faculty
-
Under Graduates
-
Post Graduates
-
Doctor of Philosophy
-
Virology Researchers
-
Faculty of Clinical trials and Research
-
Training Institutes
-
Research laboratories
-
Scientists and professors
-
Business Entrepreneurs
-
Industry professionals
-
Directors/Managers/CEO’s
-
Presidents & Vice Presidents Why to attend?